What is the Patient Access Program? The Patient Access Program, or PAP, allows a patient, the patient’s parent or legal guardian if the patient is a minor, or the patient’s legal guardian if the patient is an incapacitated person to receive a copy of the patient’s Texas Prescription Monitoring Program record.
What is the Institute for health professionals patient access specialist program?
The Institute for Health Professionals’ Patient Access Specialist program consists of 3 online, instructor-facilitated courses that will prepare you to pass the Certified Healthcare Admissions Associate (CHAA) exam. You can complete the program in 2–3 terms.
What is patient access and how does it work?
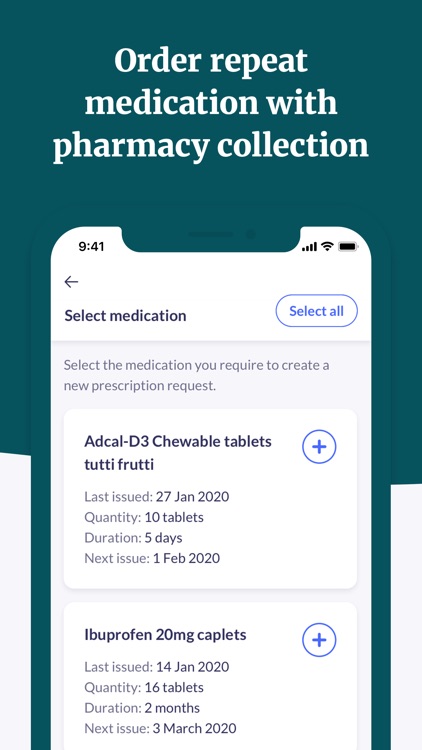
"Patient Access connects you to healthcare services when you need them most. Book GP appointments, order repeat prescriptions and explore your local pharmacy services.".
What is in partnership with patient access?
In partnership with Patient Access connects you to local health services when you need them most. Book GP appointments, order repeat prescriptions and discover local health services for you or your family via your mobile or home computer.
What is a patient access specialist (Chaa)?
This is one of the most important and concentrated positions in healthcare, encompassing many different roles and responsibilities within a hospital setting. Enroll in the Patient Access Specialist program to gain the skills and know-how to pass the Certified Healthcare Admissions Associate (CHAA) exam.

What is NHS patient access?
The NHS App and Patient Access are two online services available to patients. You will find they save you time and help you take more control of your health, particularly if you have any long-term medical conditions which require regular monitoring and frequent prescriptions.
What is EAP program in pharma?
An expanded access program (EAP) is the formal plan under which preapproval access to an investigational drug can be provided to a group of patients. Although an EAP is a regulated program, the decision to authorize an EAP is the responsibility of the biopharmaceutical sponsor.
What are managed access programs?
MAPs are programs under which investigational medicines, medicines for which a marketing authorization application is ongoing may be used to treat certain patients who cannot enroll in an ongoing clinical trial.
What are early access programs?
Early access programs are a means by which investigational therapies are made available, in certain circumstances, to treat patients with serious diseases who are unable to participate in an ongoing clinical trial or whose treatment options are otherwise limited.
What does EAP stand for in clinical trials?
Both clinical trials and Expanded Access Programs (EAPs) provide investigational new drugs (INDs) to seriously sick patients who have exhausted all viable treatment options.
What is a named patient program?
A Post-Approval Named Patient Program (PA-NPP) provides patients and physicians access to commercially approved medicines that are not available to them in their own country. These drugs must be approved in at least one country, from which it can be imported into the patient's country under a PA-NPP.
What is patient support program?
Patient Support Programs (PSPs) are an umbrella term to describe initiatives led by pharmaceutical companies to improve access, usage, and adherence to prescription drugs. These programs can have a financial component, support clinical investments, focus purely on education, or a combination.
What is the primary goal of the expanded access program?
Expanded access, also called “compassionate use,” provides a pathway for patients to gain access to investigational drugs, biologics, and medical devices used to diagnose, monitor, or treat patients with serious diseases or conditions for which there are no comparable or satisfactory therapy options available outside ...
How do I apply for compassionate drug use?
To be eligible for Right to Try, a person must:Be diagnosed with a life-threatening disease or condition.Have tried all approved treatment options for the disease or condition.Have a doctor certify that they are unable to participate in a clinical trial for the investigational drug.More items...•
What is the difference between compassionate use and emergency use?
Compassionate use means that the use does not meet the criteria for emergency use, and therefore prior FDA approval (IDE) is required before the device can be used. Standard IRB review and approval (like a research study) is required for neither emergency nor compassionate use.
What is compassionate use treatment?
Compassionate use is a treatment option that allows the use of an unauthorised medicine. Under strict conditions, products in development can be made available to groups of patients who have a disease with no satisfactory authorised therapies and who cannot enter clinical trials.
When did compassionate use start?
In the US, compassionate use started with the provision of investigational medicine to certain patients in the late 1970s, and a formal program was established in 1987 in response to HIV/AIDS patients requesting access to drugs in development.
What is an expanded access study?
Expanded access is the use of an investigational new drug outside of a clinical trial in patients for the diagnosis, monitoring, or treatment of a serious disease or condition. In contrast, participants in clinical trials/studies are considered human subjects, whether they are patients or healthy volunteers.
What is compassionate use in medicine?
Sometimes called “compassionate use”, expanded access is a potential pathway for a patient with an immediately life-threatening condition or serious disease or condition to gain access to an investigational medical product (drug, biologic, or medical device) for treatment outside of clinical trials when no comparable ...
What is Sophos early access program?
Early access programs let you try out new product features before we release them to all customers. You can take part in more than one program at the same time.
Who must make a request for a syringe?
Requests must be made by the patient, the patient’s parent or legal guardian if the patient is a minor, or the patient’s legal guardian if the patient is an incapacitated person.
What is a PAP in Texas?
The Patient Access Program, or PAP, allows a patient, the patient’s parent or legal guardian if the patient is a minor, or the patient’s legal guardian if the patient is an incapacitated person to receive a copy of the patient’s Texas Prescription Monitoring Program record.
What is patient access?
Patient Access connects you to local health services when you need them most. Book GP appointments, order repeat prescriptions and discover local health services for you or your family via your mobile or home computer.
Is patient access available in the UK?
Patient Access is now available to any UK patient. Join today and benefit from a faster, smarter way to manage your healthcare.
Multi-Stakeholder Collaboration
Leaders from across industry come together at PAP 2022 to provide fresh perspectives on the most pressing issues. Garner invaluable insights from representatives of:
Options for Learning & Networking
As a hybrid event, we're offering the options for an All-Access Pass (in-person + virtual) and Virtual-Only Pass. Plus, scholarship options are available for non-profit free healthcare clinics, advocacy groups and patient organizations.
Exclusive 2-For-1 for 2022
PAP 2022 will be co-located with Hub & Specialty Pharmacy Models East. This exclusive 2-For-1 deal provides extended and more robust learning and networking opportunities.
Acclaimed Conference Features
Any time up to 14 days before the event, you are welcome to substitute your All-Access Pass for a Virtual-Only Pass, or vice versa (pending space availability). Learn more here.
Who is the Patient Access Specialist program for?
If you are new to the healthcare industry, seeking an entry-level, front-line registration position within a hospital setting, this program may be for you.
How many terms are required for the Patient Access Specialist program?
You can complete the program in 2–3 terms. Students must take Patient Access I and Patient Access Specialist II in sequence.
Why is it important to validate patient identity?
Validating patient identity is crucial to the continuity of patient care, the reduction of patient record errors, and fraud to a hospital or care facility. Accurately identifying patients and linking them to the correct medical record is paramount to proper patient treatment. Errors due to inaccurate patient identification can lead to improper healthcare and high organizational costs.
Can you use Bridgefront for patient access?
If you are interested in purchasing whole curriculums of education for your entire Patient Access team and/or other groups of users, you can work directly with BridgeFront. Teams can utilize the Litmos Learning Management System to improve performance. Team leaders can build courses quickly, add/delete users, and create targeted learning plans for groups and individual learners. Learners can easily use their mobile devices and managers can run and share reports to monitor team learning progress. For more information, contact Terry Kile at [email protected]
Who is eligible for Novartis Managed Access programs?
Novartis considers granting managed access to investigational or pre-approval products when all of the following criteria are met:
What is Novartis Managed Access?
There are instances where a patient has a serious or life-threatening disease or condition, for which all currently available treatment options have been exhausted and enrollment into a clinical trial is not possible.
How do I submit a request for Managed Access?
A request must be submitted by the treating physician on behalf of the patient . Requests can be submitted via our portal by clicking here:
What is sufficient data?
Sufficient data exists to believe the potential benefit of treatment outweighs the potential risk in the context of the disease or condition to be treated;
Can a treating physician request an investigational or pre-approval product prior to regulatory approval?
In these cases, the treating physician can request an investigational or pre-approval product prior to regulatory approval, provided it is allowed by the applicable local laws. Within Novartis, we refer to such provision of investigational or pre-approval products as “Managed Access.”
